0 引言
㶲可用于衡量热力系工质的可用能,从“量”和“质”2个方面衡量了能量的“价值”。㶲分析以热力学第一和第二定律为依据,是估计系统中不可逆性位置和大小的一个重要工具。随着对燃料电池数学模型的持续优化,对不同燃料电池系统㶲分析的研究也在不断增多。如Chan等[21]研究了由氢气和甲烷供给的固体氧化物燃料电池系统的热力学模型,发现它们的第一定律效率(电效率)分别为50.97%和52.28%,第二定律效率(㶲效率)分别为62.19%和59.96%。Hussain等[22]建立了质子交换膜燃料电池(proton exchange membrane fuel cell,PEMFC)动力系统的热力学模型,并研究了工作温度、压力和空气化学计量比对系统㶲效率的影响,结果显示:当工作温度和压力升高时,PEMFC的㶲效率提高;但是,随着空气化学计量比的增加,㶲效率没有显著提高。Baralli等[23]分析了基于PEMFC的微热电联产系统,研究了燃料电池运行条件(相对湿度、压力和温度)对系统㶲效率的影响,结果表明:随着压力和温度的增加,系统㶲效率不断提高;随着相对湿度的增加,系统㶲效率先逐渐提高,当相对湿度大于58%时,㶲效率开始下降。
为此,本文基于热力学第一和第二定律,推导出PAFC的电效率、输出功率、㶲损率、㶲效率、生态函数和生态性能系数等性能参数的解析表达式,揭示PAFC的能效、㶲和生态特性。在此基础上,考虑多种优化目标之间的权衡,并根据不同的要求进一步细分参数优化区域。最后,分析一些重要操作条件和设计参数对PAFC性能的影响。
1 PAFC的数学模型
1.1 系统描述
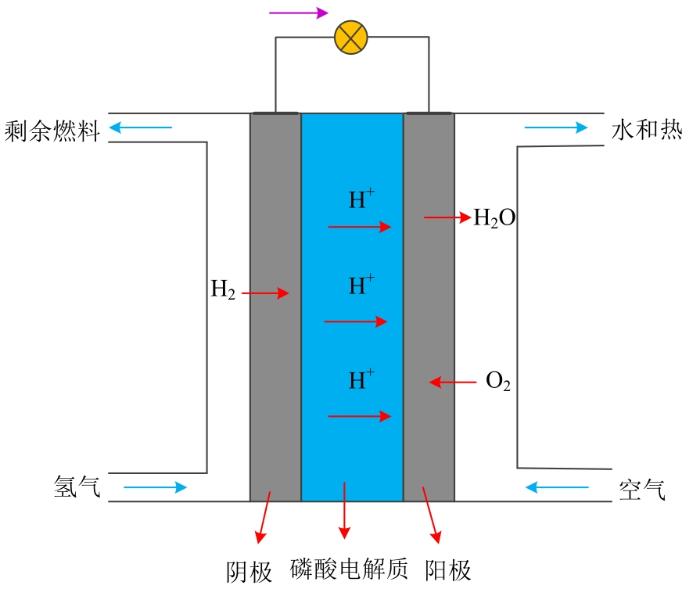
图1
值得注意的是,总反应的描述包括外能,即电能和热能。整个反应的基本热力学关系表示为
式中:
表1 H2、O2和H2O的热力学参数
Tab. 1
| i | ||||||
|---|---|---|---|---|---|---|
| H2(g) | 0 | 0 | 131 | 27.28+0.003 26T+50 000/T2 | — | 236 090 |
| O2(g) | 0 | 0 | 205 | 29.96+0.004 18T-167 000/T2 | — | 3 970 |
| H2O(g) | — | — | — | 30.00+0.010 17T+33 000/T2 | 40 700 | — |
| H2O(l) | 237 200 | 285 800 | 70 | 75.44 | — | 9 500 |
根据法拉第定律,电化学反应中的氢消耗速率表示为
式中:
因此,单位时间反应释放的总能量[37]表示为
式中摩尔焓变
PAFC的理论最大电位,即可逆电位
式中:
PAFC的实际输出电压
活化过电势是由于在三相界面驱动能量转移需要的活化能所引起的电压损失,可表示为
式中:
浓差过电势是由于电解液中的扩散或对流问题,以及反应物浓度未保持在初始水平而引起的电压损失,可表示为
式中
欧姆过电势是燃料电池通过电流时由电解液的电阻引起的电位降,可表示为
式中:
根据实验数据,Chin等[38]提出了一个方程,可以用于描述磷酸溶液的比电导率、温度、黏度和浓度之间的关系,即
式中
PAFC输出电压[39]表示为
根据式(
1.2 PAFC的能效特性分析
PAFC的输出功率
式中A为燃料电池电极板的有效面积。
从PAFC扩散到环境中的热流率表示为
式中:
根据热力学第一定律,PAFC剩余的热耗率[40]表示为
式中
1.3 PAFC的㶲特性分析
㶲是用于测量能量品位的,它被定义为系统与热力学平衡态相互作用时理论上能获得的最大功。在忽略动力㶲和势㶲的情况下,流体的㶲主要包括化学㶲
式中
物理㶲
式中:s、v、g和z 分别为熵、速度、重力和高度;
PAFC系统反应物(H2和O2)中的总输入㶲
在所有实际的不可逆过程中,能量的贬值将不可避免地发生,通常用㶲损率来描述。根据热力学第二定律,PAFC的㶲损率[44]表示为
PAFC的㶲效率反映了㶲的利用率[44],可表示为
1.4 PAFC的生态特性分析
PAFC的熵增率[45]可表示为
2 PAFC的性能特性和参数分析
2.1 PAFC系统典型参数
表2 PAFC系统的参数
Tab. 2
| 参数 | 数值 | 参数 | 数值 |
|---|---|---|---|
| F/(C·mol-1) | 96 485 | 0.000 03 | |
| R/(J·mol-1·K-1) | 8.314 | 0.000 8 | |
| 2 | α | 0.5 | |
| p/Pa | 101 325 | j0/(A·m-2) | 0.06 |
| T/K | 453 | tele/m | 0.002 |
| T0/K | 298 | A/m2 | 0.001 5 |
2.2 能效、㶲和生态特性
由式(
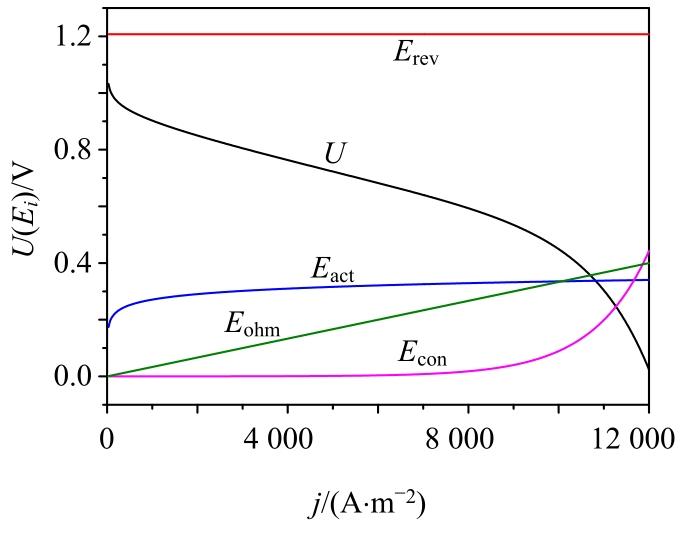
图2
图2
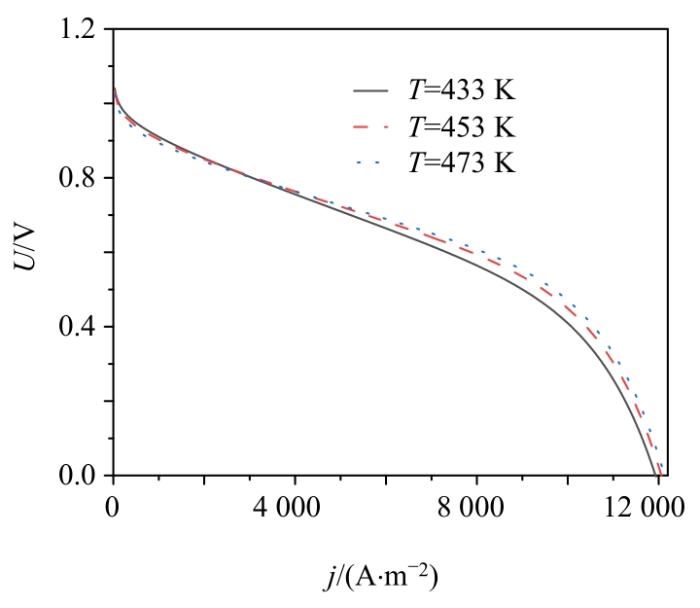
Erev, Econ, Eact, Eohm, U随j变化曲线
Fig. 2
Change curves of Erev, Econ, Eact, Eohm and U with j
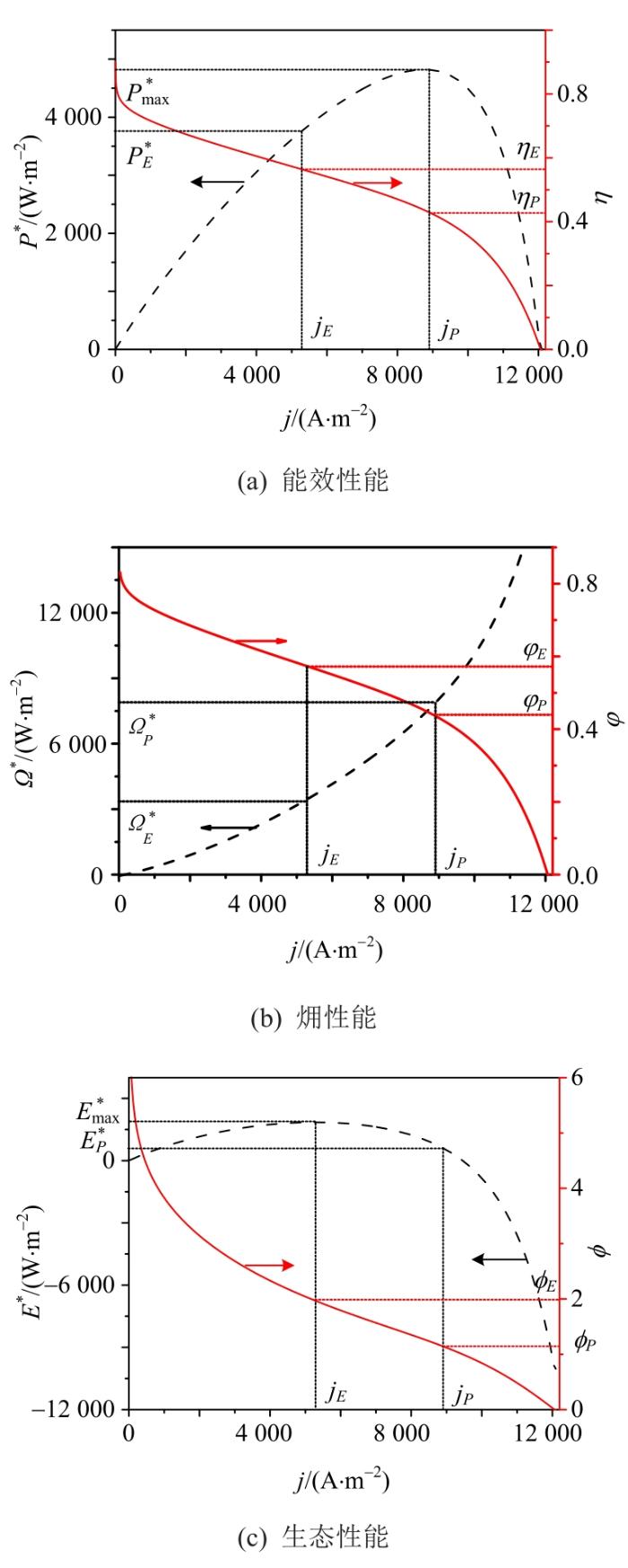
功率密度P* 、电效率
图3
表3 一些关键性能参数的比较
Tab. 3
| i | ||||
|---|---|---|---|---|
| 增率/% | 29.3 | -55.1 | 29.1 | 66.7 |
| P | 43.7 | 7 629.1 | 44.4 | 1.2 |
| E | 56.5 | 3 425.5 | 57.3 | 2.0 |
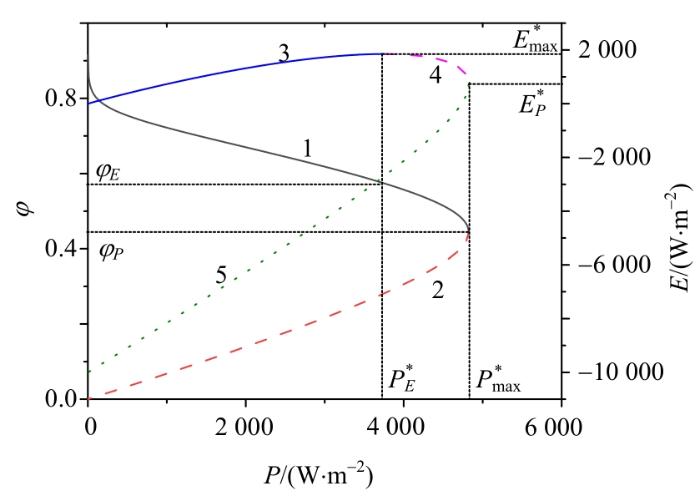
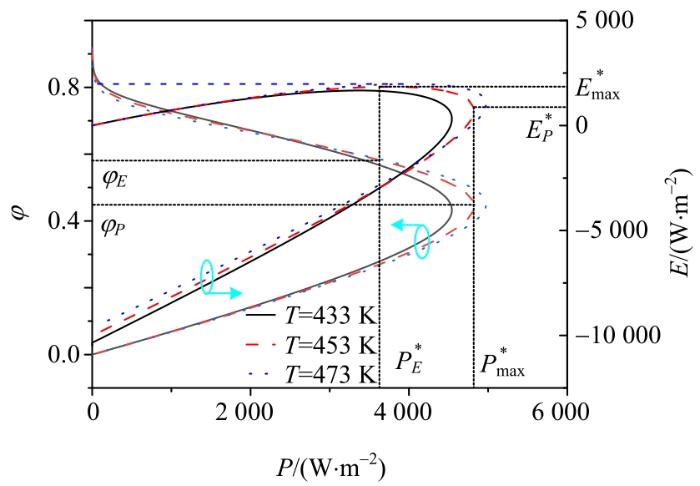
通过考虑多种优化目标之间的权衡,可获得不同的优化区域。图4给出了PAFC的㶲效率(曲线1、2)和生态函数密度(曲线3、4、5)随功率密度的变化情况。在曲线1,㶲效率
图4
图4
㶲效率和生态函数密度随功率密度的变化情况
Fig. 4
Variations of exergy efficiency and ecology function density
由以上分析可知,当考虑效能和生态性能之间的平衡时,最佳区域应位于
2.3 参数分析
由以上分析可知,PAFC性能与一些重要的工作条件和设计参数有关,如工作温度、工作压力、电解质厚度和交换电流密度。
2.3.1 工作温度的影响
随着工作温度T的增加,欧姆过电势Eohm、可逆电位
图5
图6
图6
工作温度T对输出功率密度、㶲效率和生态函数密度的影响Fig. 6 Effect of working temperature T on output power density, exergy efficiency and ecology function density
2.3.2 工作压力的影响
图7
图7
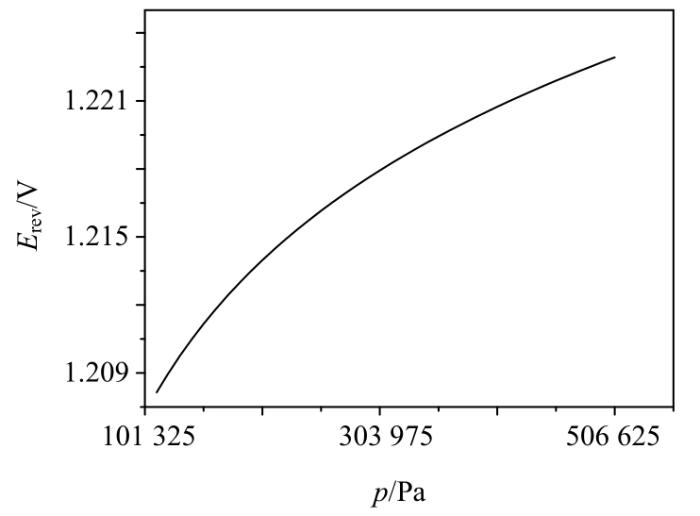
工作压力p对可逆电位Erev的影响
Fig. 7
Effect of operating pressure p on reversible potential Erev
图8
图8
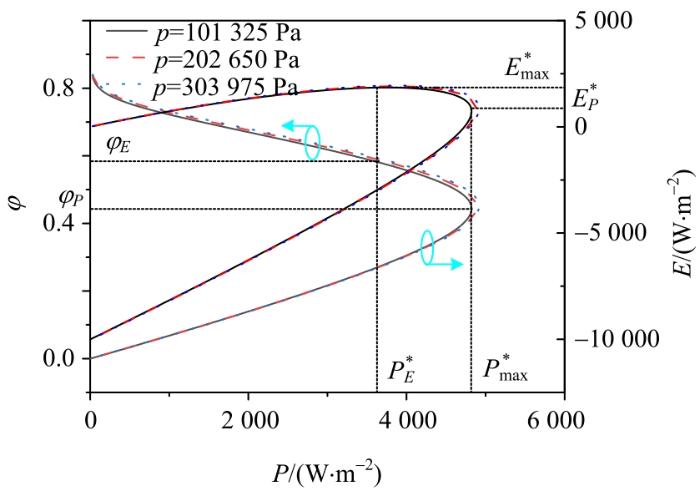
工作压力p对输出功率密度、㶲效率和生态函数密度的影响
Fig. 8
Effect of operating pressure p on output power density, exergy efficiency and
综上所述,与工作温度类似,增加PAFC的工作压力有利于提高系统的性能,但后者对性能的影响相对较小。此外,增加工作压力也会消耗额外的能量来压缩入口反应物,导致设备成本、尺寸和重量增加,实际上,
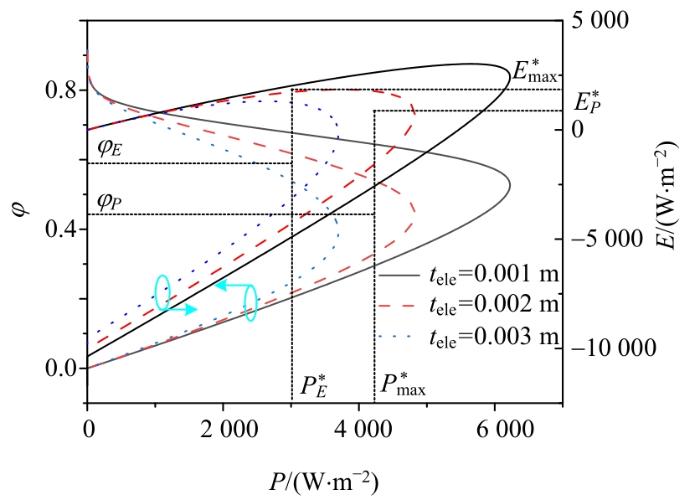
2.3.3 电解质厚度的影响
由
图9
图9
电解质厚度tele对输出功率密度、㶲效率和生态函数密度的影响
Fig. 9
Effect of electrolyte thickness tele on output power density, exergy efficiency andecology function density
由以上分析可知,减小电解质厚度
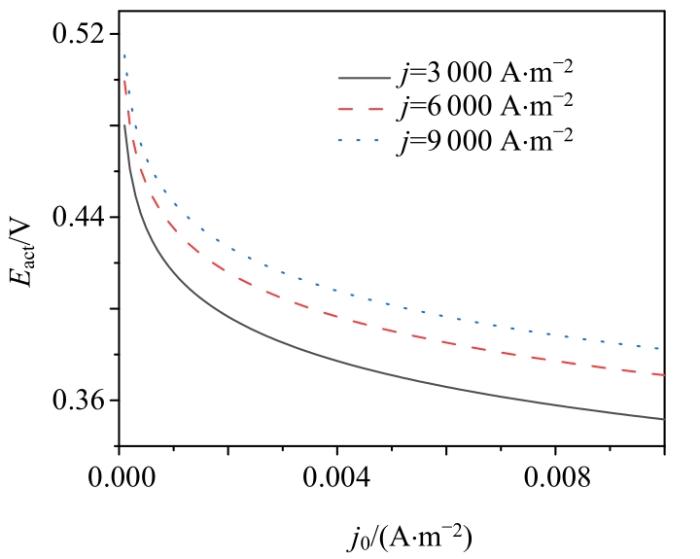
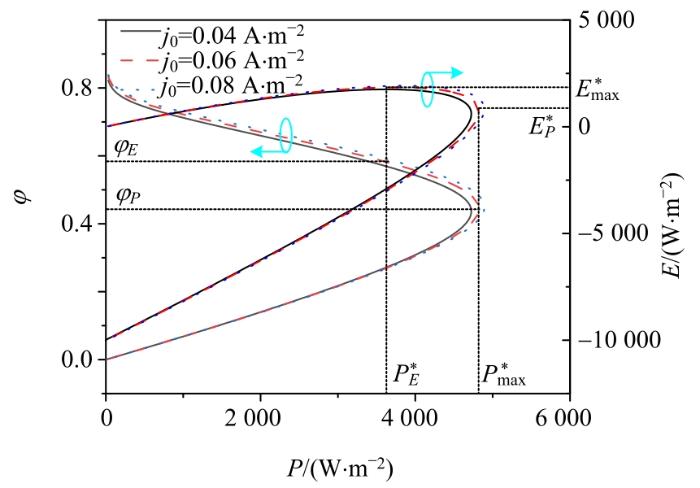
2.3.4 交换电流密度的影响
交换电流密度
图10
图10
交换电流密度j0对活化过电势Eact的影响
Fig. 10
Effect of exchange current density j0 on activation overpotential Eact
图11
图11
交换电流密度j0对输出功率密度、㶲效率和生态函数密度的影响
Fig. 11
Effect of exchange current density j0 on output power density, exergy efficiency and ecology function density
3 结论
PAFC因其工作温度适中、耐用性高和结构简单等优点而成为商业化发展最快的一种燃料电池,然而它也存在功率密度低、寿命短和制造成本高等缺点,阻碍了其进一步开发与应用。为了完善PAFC优化设计理论,研究了PAFC的能效、㶲和生态性能特性,主要结论如下:
1)PAFC的最大功率密度和最大生态函数密度分别在jP =8 750.8 A/m2和jE =5 260.8 A/m2时获得,其值分别为48 822.4 W/m2和1 850.0 W/m2。
2)当功率密度处于最大值时,PAFC的电效率、㶲损率密度、㶲效率和生态性能系数的参数值均明显小于当生态函数密度处于最大值时以上参数对应的值。
3)减小电解质厚度、提高工作温度、增加工作压力、增大交换电流密度均有利于改善PAFC系统性能。此外,在这些参数的合理有效范围内,工作温度和电解质厚度对PAFC性能的影响比工作压力和交换电流密度的影响更加显著。
参考文献
燃料电池用聚合物质子交换膜的研究进展
[J].
Research progresses in polymeric proton exchange membranes for fuel cells
[J].
Optimal design and economic analysis of a hybrid solid oxide fuel cell and parabolic solar dish collector,combined cooling,heating and power (CCHP) system used for a large commercial tower
[J].
适合分布式冷热电联供系统的中小型发电装置
[J].
Small and medium-scale power generation devices suiting for distributed combined cooling, heating and power system
[J].
燃料电池汽车基本技术及发展综述
[J].
Review on basic technology and development of fuel cell vehicle
[J].
一种适用于燃料电池-超级电容发电系统的控制策略
[J].
A control strategy for fuel cell-ultracapacitor power generation system
[J].
Thermo-economic analysis of phosphoric acid fuel-cell (PAFC) integrated with organic ranking cycle (ORC)
[J].
Economic and environmental assessment of phosphoric acid fuel cell-based combined heat and power system for an apartment complex
[J].
Prospects of fuel cell technologies
[J].
Effects of flow rate and starvation of reactant gases on the performance of phosphoric acid fuel cells
[J].
Characterization and performance analysis of silicon carbide electrolyte matrix of phosphoric acid fuel cell prepared by ball-milling method
[J].
A high-performance phosphoric acid fuel cell
[J].
Properties of Pt/C catalyst modified by chemical vapour deposition of Cr as a cathode of phosphoric acid fuel cell
[J].
Predicting the effects of process parameters on the performance of phosphoric acid fuel cells using a 3-D numerical approach
[J].
Effect of surface groups on the electro catalytic behaviour of Pt-Fe-Co alloy-dispersed carbon electrodes in the phosphoric fuel cell
[J].
A fuel cell operating between room temperature and 250 ℃ based on a new phosphoric acid based composite electrolyte
[J].
A two dimensional steady-state model for phosphoric acid fuel cells (PAFC)
[J].
Development of a 1 kW phosphoric acid fuel cell stack
[J].
Electrical characterization of a 2.5 kW phosphoric acid fuel cell stack operating on simulated reformed biogas
[J].
Development of prototype phosphoric acid fuel cell pick-up electric vehicle
[C]//
Investigation of phosphoric acid fuel cell, linear Fresnel solar reflector and organic Rankine cycle polygeneration energy system in different climatic conditions
[J].
Energy and exergy analysis of simple solid-oxide fuel-cell power systems
[J].
Thermodynamic analysis of a PEM fuel cell power system
[J].
An energetic/exergetic analysis of a residential CHP system based on PEM fuel cell
[J].
An ecological optimization criterion for finite-time heat engines
[J].
Ecological optimization and performance study of irreversible Stirling and Ericsson heat engines
[J].
Ecological optimization of an irreversible Brayton heat engine
[J].
Ecological optimization of quantum spin-1/2 heat engine at the classical limit
[J].
Ecological analysis of a thermally regenerative electrochemical cycle
[J].
Energetic,exergetic and ecological analyses of a high-temperature proton exchange membrane fuel cell based on a phosphoric- acid-doped polybenzimidazole membrane
[J].
Performance analyses of an integrated phosphoric acid fuel cell and thermoelectric device system for power and cooling cogeneration
[J].
Evaluation of the high temperature electrolysis of steam to produce hydrogen
[J].
Performance analysis and multiobjective optimization of a new molten carbonate fuel cell system
[J].
Maximum power output and load matching of a phosphoric acid fuel cell-thermoelectric generator hybrid system
[J].
Performance of a combined system consisting of a high-temperature polymer electrolyte fuel cell and an absorption refrigerator
[J].
Parametric investigation of phosphoric acid fuel cell-Thermally regenerative electro chemical hybrid system
[J].
Optimal current paths for model electrochemical system
[J].
Multi-objective optimisation analysis and load matching of a phosphoric acid fuel cell system
[J].
On the conductivity of phosphoric acid electrolyte
[J].
A study of double functions and load matching of a phosphoric acid fuel cell/heat-driven refrigerator hybrid system
[J].
Performance assessment of phosphoric acid fuel cell-thermoelectric generator hybrid system with economic aspect
[J].
Exergy analysis of an integrated solid oxide fuel cell and organic Rankine cycle for cooling,heating and power production
[J].
Energy and exergy utilization in agricultural sector of Saudi Arabia
[J].
Energy and exergy analyses of a biomass trigeneration system using an organic Rankine cycle
[J].
Ecological coefficient of performance analysis and optimization of an irreversible regenerative-Brayton heat engine
[J].
Performance analysis and optimization of an irreversible dual-cycle based on an ecological coefficient of performance criterion
[J].
A high-yield and ultra-low-temperature methanol reformer integratable with phosphoric acid fuel cell (PAFC)
[J].
Influence of CO concentration and reactant gas pressure on cell performance in PAFC
[J].
Optimizing polymer electrolyte membrane thickness to maximize fuel cell vehicle range
[J].