

发电技术 ›› 2022, Vol. 43 ›› Issue (4): 609-617.DOI: 10.12096/j.2096-4528.pgt.22002
张欢1, 汪丽1, 叶舣1,2, 赵兴雷2
收稿日期:2022-01-14
出版日期:2022-08-31
发布日期:2022-09-06
作者简介:基金资助:Huan ZHANG1, Li WANG1, Yi YE1,2, Xinglei ZHAO2
Received:2022-01-14
Published:2022-08-31
Online:2022-09-06
Supported by:摘要:
为解决传统吸收剂的CO2捕集工艺存在的再生能耗高及捕集效率低等问题,新型高效低能耗溶剂的研发已成为当前研究的重点。用二乙烯三胺(DETA)作为主体吸收剂,三乙醇胺(TEA)为辅助吸收剂,按总胺质量分数为30%的不同配比混合,以传统吸收剂单乙醇胺(MEA)质量分数30%作参考标准,进行CO2吸收-解吸性能、解吸能耗及黏度测试研究,筛选出DETA+TEA吸收效果最优的配比。实验结果表明,当质量分数分别为20%、10%的DETA和TEA,不仅具有较高的CO2吸收容量,而且具有优异的再生性能和较低的解吸能耗。该吸收剂饱和CO2吸收量为3.71 mol/L溶剂,最大解吸速率为1.94 mmol/(L?min),解吸能耗为160 kJ/mol,与质量分数30%MEA吸收剂相比,饱和吸收量提高了34.42%,最大解吸速率提高了170%,能耗降低了21.2%。20次循环稳定性高达98%,吸收剂使用黏度为3.1~6.88 mPa·s。
中图分类号:
张欢, 汪丽, 叶舣, 赵兴雷. 乙二烯三胺与三乙醇胺混合胺溶液CO2吸收剂研究[J]. 发电技术, 2022, 43(4): 609-617.
Huan ZHANG, Li WANG, Yi YE, Xinglei ZHAO. Study on CO2 Absorbent of DETA and TEA Mixed Amine Solution[J]. Power Generation Technology, 2022, 43(4): 609-617.
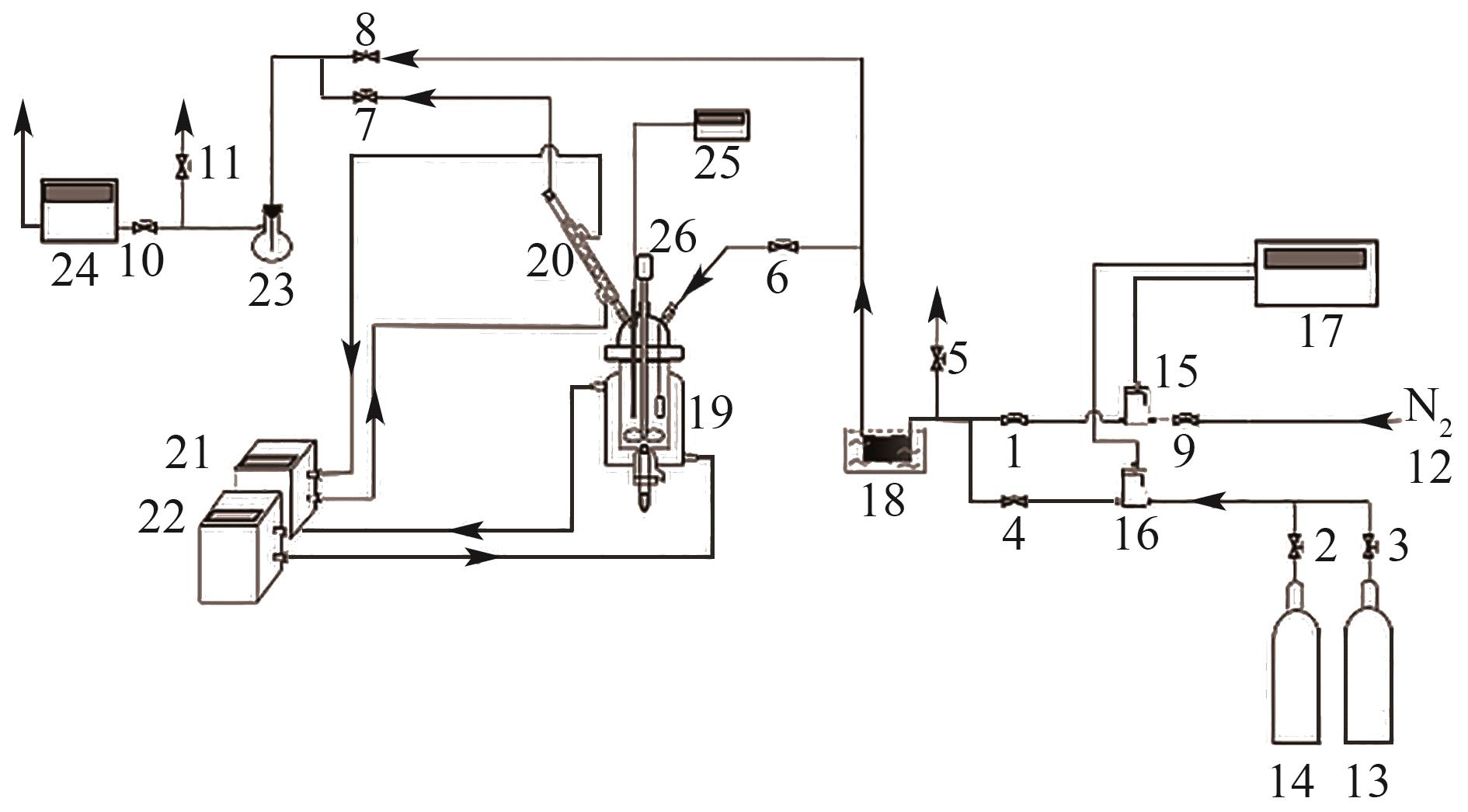
图1 实验装置示意图1, 2, …, 11—阀门;12—N2进口;13—CO2气瓶;14—15% CO2标准气瓶;15—N2气体流量控制计;16—CO2气体流量控制计;17—气体流量显示仪;18—恒温水浴锅;19—常压反应釜;20—球形冷凝管;21—DC1006节能型智能恒温槽;22—HH-501BS循环恒温油浴锅;23—集水瓶;24—煤气分析仪;25—温度记录仪;26—电动搅拌机。
Fig. 1 Experimental setup diagram
| 参数 | 30%MEA | 20%DETA+10%TEA |
|---|---|---|
| 30% | 30% | |
| Δt/℃ | 60 | 60 |
| Δx/(mol CO2/mol胺) | 0.5 | 1.36 |
| c/[kJ/(kg·K)] | 3.236 | 2.365 |
| M/(kg/mol CO2) | 0.061 | 0.115 |
| ΔHads/(kg/mol CO2) | 90 | 80 |
| qg/(kg/mol CO2) | 58.5 | 40.39 |
| Qrs/(kg/mol CO2) | 54.56 | 39.51 |
| W/(kg/mol CO2) | 203 | 160 |
表1 解吸能耗数据
Tab. 1 Desorption energy consumption calculation data
| 参数 | 30%MEA | 20%DETA+10%TEA |
|---|---|---|
| 30% | 30% | |
| Δt/℃ | 60 | 60 |
| Δx/(mol CO2/mol胺) | 0.5 | 1.36 |
| c/[kJ/(kg·K)] | 3.236 | 2.365 |
| M/(kg/mol CO2) | 0.061 | 0.115 |
| ΔHads/(kg/mol CO2) | 90 | 80 |
| qg/(kg/mol CO2) | 58.5 | 40.39 |
| Qrs/(kg/mol CO2) | 54.56 | 39.51 |
| W/(kg/mol CO2) | 203 | 160 |
| 1 | 李国志 .基于技术进步的中国低碳经济研究[D].南京:航空航天大学,2011. |
| LI G Z .Research on China’s low-carbon economy based on technological progress[D].Nanjing:University of Aeronautics and Astronautics,2011. | |
| 2 | RODHE H .A comparison of the contribution of various gases to the greenhouse effect[J].Science,1990,248(4960):1217-1219. doi:10.1126/science.248.4960.1217 |
| 3 | SAVARESI A .The Paris Agreement: a new beginning?[J].Journal of Energy & Natural Resources Law,2016,34(1):16-26. doi:10.1080/02646811.2016.1133983 |
| 4 | 邹才能,赵群,张国生,等 .能源革命:从化石能源到新能源[J].天然气工业,2016,36(1):1-10. doi:10.1016/j.ngib.2016.02.001 |
| ZHOU C N, ZHAO Q, ZHANG G S,et al .Energy revolution: From a fossil energy era to a new energy era[J].Natural Gas Industry,2016,36(1):1-10. doi:10.1016/j.ngib.2016.02.001 | |
| 5 | 谢国辉,李娜娜,元博 .我国新能源开发路线图分析方法及模型[J].发电技术,2020,41(6):631-637. doi:10.12096/j.2096-4528.pgt.19174 |
| XIE G H, LI N N, YUAN B .Analysis methods and model of new energy developing roadmap in China[J].Power Generation Technology,2020,41(6):631-637. doi:10.12096/j.2096-4528.pgt.19174 | |
| 6 | ELMEKAWY A, HEGAB H M, MOHANAKRISHNA G,et al .Technological advances in CO2 conversion electro-biorefinery:a step toward commercialization[J]. Bioresource Technology,2016,215:357-370. doi:10.1016/j.biortech.2016.03.023 |
| 7 | 朱维群,王倩 .碳中和目标下的化石能源利用新技术路线开发[J].发电技术,2021,42(1):3-7. doi:10.12096/j.2096-4528.pgt.21009 |
| ZHU W Q, WANG Q .Development of New technological routes for fossil energy utilization under the goal of carbon neutra[J].Power Generation Technology,2021,42(1):3-7. doi:10.12096/j.2096-4528.pgt.21009 | |
| 8 | AJANOVIC A .Renewable fuels:a comparative assessment from economic,energetic and ecological point-of-view up to 2050 in EU-countries[J].Renewable Energy,2013,60:733-738. doi:10.1016/j.renene.2013.06.012 |
| 9 | SHEN J, KAUR I, BAKTASH M M,et al .A combined process of activated carbon adsorption,ion exchange resin treatment and membrane concentration for recovery of dissolved organics in pre-hydrolysis liquor of the kraft-based dissolving pulp production process[J].Bioresource Technology,2013,127:59-65. doi:10.1016/j.biortech.2012.10.031 |
| 10 | 王清 .化学吸收法捕集烟气中二氧化碳的研究综述[J].科技经济导刊,2021(12):126-127. |
| WANG Q .A review of the research on capture of carbon dioxide in flue gas by chemical absorption[J].Guide to Science and Technology Economics,2021(12):126-127. | |
| 11 | WANG S, AN C, ZHANG Q H .Syntheses and structures of lithium zirconates for high-temperature CO2 absorption[J].Journal of Materials Chemistry A,2013,1(11):3540-3550. doi:10.1039/c2ta00700b |
| 12 | LITTEL R J, VERSTEEG G F, VAN SWAAIJ W P M .Kinetics of CO2 with primary and secondary amines in aqueous solutions:Ⅱ.Influence of temperature on zwitterion formation and deprotonation rates[J].Chemical Engineering Science,1992,47(8):2037-2045. doi:10.1016/0009-2509(92)80320-c |
| 13 | MURUGESAN K, SENTHAMARAI T, CHANDRASHEKHAR V G,et al .Catalytic reductive aminations using molecular hydrogen for synthesis of different kinds of amines[J].Chemical Society Reviews,2020,49(17):6273-6328. doi:10.1039/c9cs00286c |
| 14 | LI K, FANG H, DUAN X,et al .Efficient uptake of NH3 by dual active sites NH4SCN-imidazole deep eutectic solvents with low viscosity[J].Journal of Molecular Liquids,2021,339:116724. doi:10.1016/j.molliq.2021.116724 |
| 15 | ROSLI A, AHMAD A L, LIM J K,et al .Advances in liquid absorbents for CO2 capture:a review[J].Journal of Physical Science,2017,28:121-141. |
| 16 | OCHEDI F O, YU J, YU H,et al .Carbon dioxide capture using liquid absorption methods:a review[J].Environmental Chemistry Letters,2021,19(1):77-109. doi:10.1007/s10311-020-01093-8 |
| 17 | GALINDO P, SCHAFFER A, BRECHTEL K,et al .Experimental research on the performance of CO2-loaded solutions of MEA and DEA at regeneration conditions[J].Fuel,2012,101:2-8. doi:10.1016/j.fuel.2011.02.005 |
| 18 | GAO H, WU Z, LIU H,et al .Experimental studies on the effect of tertiary amine promoters in aqueous monoethanolamine (MEA) solutions on the absorption/stripping performances in post -combustion CO2 capture[J].Energy & Fuels,2017,31(12):13883-13891. doi:10.1021/acs.energyfuels.7b02390 |
| 19 | ISLAM M S, YUSOFF R, ALI B S,et al .Degradation studies of amines and alkanolamines during sour gas treatment process[J].International Journal of Physical Sciences,2011,6(25):5877-5890. |
| 20 | NWAOHA C, SAIWAN C, SUPAP T,et al .Carbon dioxide (CO2) capture performance of aqueous tri-solvent blends containing 2-amino-2-methyl-1-propanol (AMP) and methyldiethanolamine (MDEA) promoted by diethylenetriamine (DETA)[J].International Journal of Greenhouse Gas Control,2016,53:292-304. doi:10.1016/j.ijggc.2016.08.012 |
| 21 | CONWAY W, BRUGGINK S, BEYAD Y,et al .CO2 absorption into aqueous amine blended solutions containing monoethanolamine (MEA),N,N-dimethylethanolamine (DMEA),N,N-diethylethanolamine (DEEA) and 2-amino-2-methyl-1-propanol (AMP) for post-combustion capture processes[J].Chemical Engineering Science,2015,126;446-454. doi:10.1016/j.ces.2014.12.053 |
| 22 | CHATTOPADHYAY D K, WEBSTER D C .Thermal stability and flame retardancy of polyurethanes[J].Progress in Polymer Science,2009,34(10):1068-1133. doi:10.1016/j.progpolymsci.2009.06.002 |
| 23 | BERTINI F, CANETTI M, PATRUCCO A,et al .Wool keratin-polypropylene composites:properties and thermal degradation[J].Polymer Degradation and Stability,2013,98(5):980-987. doi:10.1016/j.polymdegradstab.2013.02.011 |
| 24 | MA D, ZHU C, FU T,et al .An effective hybrid solvent of MEA/DEEA for CO2 absorption and its mass transfer performance in microreactor[J].Separation and Purification Technology,2020,242:116795. doi:10.1016/j.seppur.2020.116795 |
| 25 | GUO H, LI C, SHI X,et al .Nonaqueous amine-based absorbents for energy efficient CO2 capture[J].Applied Energy,2019,239:725-734. doi:10.1016/j.apenergy.2019.02.019 |
| 26 | LIN Y J, ROCHELLE G T .Approaching a reversible stripping process for CO2 capture[J].Chemical Engineering Journal,2016,283:1033-1043. doi:10.1016/j.cej.2015.08.086 |
| 27 | WANG X, CHEN L, GUO Q .Development of hybrid amine-functionalized MCM-41 sorbents for CO2 capture[J].Chemical Engineering Journal,2015,260:573-581. doi:10.1016/j.cej.2014.08.107 |
| 28 | 张克舫,刘中良,王远亚,等 .化学吸收法CO2捕集解吸能耗的分析计算[J].化工进展,2013,32(12):3008-3014. |
| ZHANG K F, LIU Z L, WANG Y Y .Analysis and calculation of the desorption of CO2 capture process by chemical absorption method[J].Chemical Industry and Engineering Progress,2013,32(12):3008-3014. | |
| 29 | GAO J, WANG S, ZHAO B,et al .Pilot-scale experimental study on the CO2 capture process with existing of SO2:degradation,reaction rate,and mass transfer[J].Energy & Fuels,2011,25(12):5802-5809. doi:10.1021/ef2010496 |
| [1] | 孙宇航, 李超, 王争荣, 孙路长, 王凯亮, 胡昔鸣, 方梦祥, 张锋. 甲基二乙醇胺-二元胺混合体系烟气CO2吸收再生性能研究[J]. 发电技术, 2024, 45(3): 468-477. |
| [2] | 翟融融, 魏清, 冯凌杰, 孙舸洵. 耦合膜冷凝器的碳捕集系统能耗特性分析[J]. 发电技术, 2023, 44(5): 667-673. |
| [3] | 汪丽, 张欢, 叶舣, 赵兴雷. N-氨乙基哌嗪与甘氨酸钠CO2吸收剂配方研究[J]. 发电技术, 2023, 44(5): 674-684. |
| [4] | 胡道成, 王睿, 赵瑞, 孙楠楠, 徐冬, 刘丽影. 二氧化碳捕集技术及适用场景分析[J]. 发电技术, 2023, 44(4): 502-513. |
| [5] | 赵连鹏, 张振扬, 安刚, 杨申音. 混合冷剂氢液化技术研究进展[J]. 发电技术, 2023, 44(3): 331-339. |
| [6] | 李小端, 赵兴雷, 叶舣, 王保登, 熊日华. 基于电渗析技术的CO2吸收剂再生过程研究[J]. 发电技术, 2022, 43(4): 593-599. |
| [7] | 王焕君, 刘牛, 郑棹方, 邢侠, 郜时旺, 刘练波, 牛红伟, 郭东方. 直接空气捕碳材料研究进展[J]. 发电技术, 2022, 43(4): 533-543. |
| [8] | 李源, 郭志成, 孟晓超, 陈科峰, 任利明, 毛睿, 岑可法. 基于可调谐二极管激光吸收光谱技术的炉内燃烧场参数在线监测系统设计[J]. 发电技术, 2022, 43(2): 353-361. |
| [9] | 薛凯, 王义函, 陈衡, 徐钢, 雷兢. 槽式太阳能辅助生物质热电联产系统热力学性能分析[J]. 发电技术, 2021, 42(6): 653-664. |
| [10] | 邱国华,魏宏鸽,梁秀进,李壮,王丰吉,朱跃. 火电机组脱硫超低排放运行能耗分析与节能运行展望[J]. 发电技术, 2020, 41(5): 510-516. |
| [11] | 张俊博,金旭,刘忠彦,车德勇,隋军. 吸收式热泵余热回收先进技术综述[J]. 发电技术, 2020, 41(3): 269-280. |
| [12] | 黄平瑞,周震,魏高升,杜小泽. 基于多孔陶瓷的体吸收太阳能集热器性能分析[J]. 发电技术, 2019, 40(1): 83-90. |
| [13] | 李文鼎,高惠华,蔡文丰. 石灰石–石膏湿法脱硫吸收塔结垢分析及预防措施[J]. 发电技术, 2019, 40(1): 51-55. |
| [14] | 梁捷, 黄柯颖. 基于家庭用电场景灵敏度分析的空调能耗研究[J]. 发电技术, 2017, 38(6): 85-89. |
| [15] | 魏宏鸽, 张杨, 杜振, 朱跃. 燃煤机组超低排放改造对机组能耗增加的影响分析及节能优化措施探讨[J]. 发电技术, 2017, 38(6): 14-17,33. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||